INTRODUCTION
The world continues to suffer from health emergencies, where human-animal interface has exacerbated and sustained the transmission of zoonotic diseases particularly in most parts of the African region.1 Lassa fever is one such zoonotic disease that has remained endemic in West Africa, affecting around 58 million people worldwide.1,2 Lassa virus is a significant cause of morbidity and mortality in resource-poor populations, accounting for 1-3 million infections and 5000 documented fatalities annually, although its overall impact in West Africa has not been ascertained.1,3 According to Coyle,2 Lassa fever is one of the top eight emerging infectious diseases that could lead to major epidemics, and outbreaks continue to pose a severe public health threat to the world. Due to its high transmission and fatality rates, Lassa fever is categorized as a category A agent, representing agents that pose the greatest risk, with a grave possibility of being used a biological weapon.3,4
The World Health Organization1 defines Lassa fever as an acute viral haemorrhagic illness caused by the Lassa virus, a member of the arenavirus family. The virus can be transmitted to humans either directly though contact with infected multimammate mice or their fluids (urine, saliva or faeces), or indirectly though contact with contaminated water, food or surfaces, or through person-person transmission via bodily fluids.1,3 Some of the common signs and symptoms following infection with the virus include fever, headaches, tiredness, and stomach pain, which can range in severity from mild to fatal haemorrhagic fever.4
Lassa fever is endemic in Nigeria, Ghana, Guinea, Liberia, Mali, Sierra Leone, Togo, and Benin.1 Recent studies show that while other countries have high rates of Lassa fever morbidity and mortality, Sierra Leone is the worst affected.5 Since the 2012 - 2013 outbreaks to date, the Eastern region of Sierra Leone has become a Lassa fever hotspot with highest incidence rates and a case fatality rate of 38.8%, particularly in Kailahun and Kenema districts.6
In these affected areas as elsewhere, Lassa fever response has mainly involved clinical case management (including case detection, treatment and infection control), and risk communication and community engagement to promote behaviour change for prevention and social supportive care.7 Current socio-cultural research data associated with risk factors and determinants of Lassa fever transmission in Eastern Sierra Lione remains crucial, yet scant.8,9 These contextual insights on risk factors and determinants of Lassa fever transmission are required to inform effective behaviour change efforts towards achieving sustained prevention and complete transmission interruption. The continued incidence of the disease despite the existing sound clinical control measures would justify the need for further research to generate contextual insights to inform reprogramming of behaviour change intervention approaches.
METHODS
Study design and setting
This was a descriptive cross-sectional quantitative study.
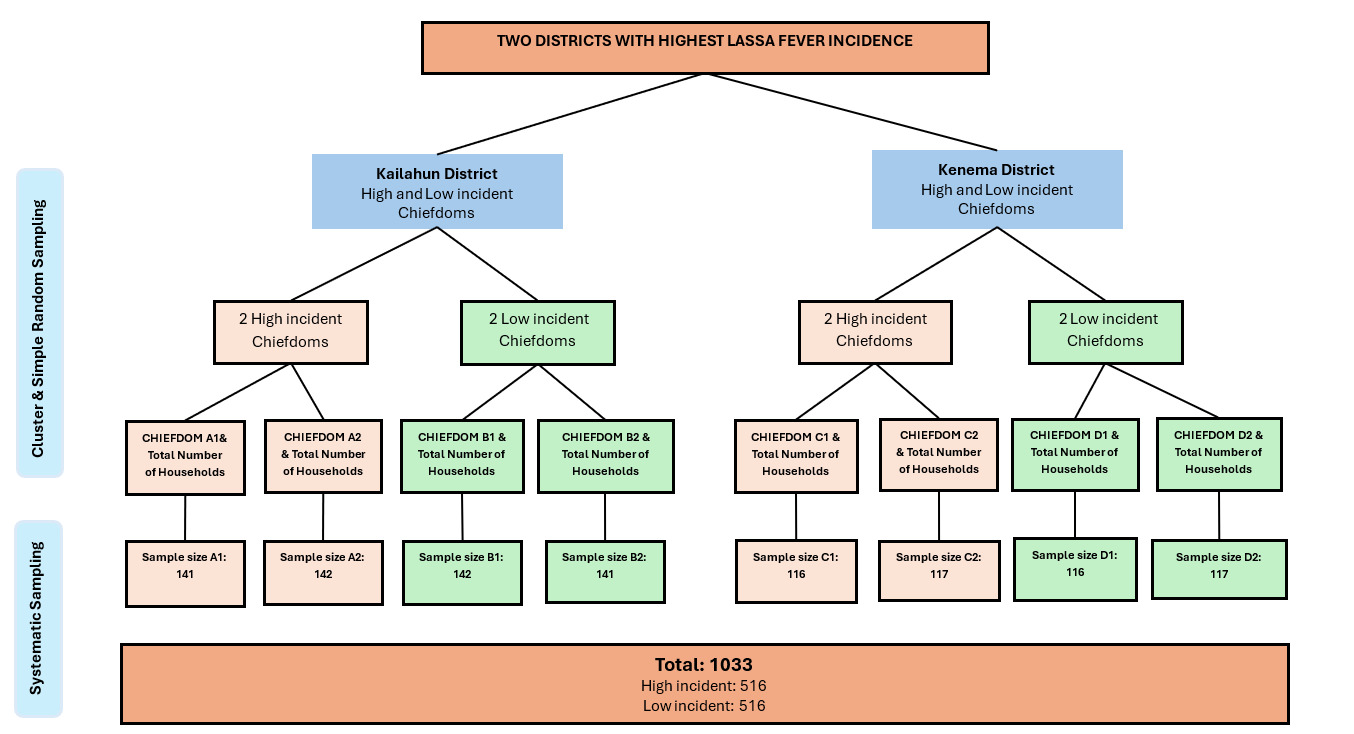
The study areas were Kailahun (land size: 4, 859 km2) and Kenema (land size: 6, 053 km2) districts, representing two of the three districts in Eastern Siera Leone.10 The characteristic forested environment and high annual rainfall in this region provides a favourable habitat for the multimammate rats that are natural reservoir hosts for the Lassa virus.10 Sierra Leone has a total of 14 districts, 11 of which are known to be endemic for Lassa fever, including the two study districts, Kailahun and Kenema, where highest transmission and incidence rates are recorded.11,12 The only known specialist isolation wards for Lassa fever in the world are strategically located in Kenema district, due to the persistently high incidence rates. With regards to demography, Mende ethnic group make up the majority of the Kailahun and Kenema districts, with Muslim as the main religion, followed by Christianity. The remaining ethnic groups include the Kissi, Vai, Fula and the Kroo.
Population and sampling
The study sample size was calculated using Slovin’s Formula13: n = where: n = sample size of households; N = population size (total number of households); e = margin of error (as a decimal), set at 0,05 (Uakarn et al., 2021). N (total number of households in the study area) was 78700 households. The researcher substituted these values in Yamane’s formula to obtain the value of n as follows: n = 78700/ [1+78700(0.052)], that is 398, which was doubled to 796, to cater for the cluster effect. The final sample size comprised of 1033 household heads as participants, considering a 30% allowance for non-response and missing data.
The questionnaire survey targeted household heads ordinarily resident in high and low incident chiefdoms of the two study districts. Participant selection was done in stages, starting with stratified sampling to select 2 high incident and 2 low incident chiefdoms per district, resulting in a total of 8 chiefdoms (4 high incident and 4 low incident chiefdoms from 2 districts). This was followed by determination of the number of households in each of the 8 chiefdoms, from the district census data. Number of participating households per chiefdom was proportionately determined based on the proportion of households in each of the 8 chiefdoms with respect to the calculated sample size as shown in Figure 1.
Data collection and participant selection criteria
Ethical clearance was obtained from the Ethics and Scientific Committee under the government of Siera Leone. Prior to data collection, the questionnaire intended for use was pretested in Moyamba district among 20 adults and revised according to pretest findings. Informed consent was sought from each potential participant, following which data was collected using an interviewer-administered electronic questionnaire in KoBoCollect® installed in android tablets, targeting household heads. The questionnaire survey collected quantitative data on risk factors and determinants of Lassa fever transmission and prevention during the dry season, a period characterised by increased contact between primary reservoirs of the virus (the rodents) and humans, thereby heightening the risk of transmission.7 Data was collected by trained research assistants who were English and native language speakers (predominantly Mende and Krio), and interpreters were consulted whenever required.
Data management and analysis
Data cleaning was performed in Microsoft Excel that allowed detection and correction of errors and inconsistences within the data. Missing data was handled by data verification at point of data collection; mean/median or regression imputation or listwise deletion, during data analysis, as appropriate. Descriptive data analysis was done in IBM SPSS software version 29.0.2.0(20) ®, summarizing the data as frequencies, proportions, means and standard deviations, and the results were presented using tables, graphs and pie charts as appropriate. Tests of associations and relationships between study variables were performed in SPSS®.
RESULTS
Socio-demographic information
A total of 1033 respondents participated in the survey as shown in Table S1 in the Online Supplementary Document. More than half of the respondents were from Kailahun District (54.8%). Respondents aged 30-39 were the largest group (29.6%). A higher proportion of the respondents were females (58.1%), and most (67.8%) were married. Most respondents were farmers (48.5%), and the highest proportion had a household size of 5-8 (49.6%). The majority of 531 respondents with formal education had attained secondary education (65.9%), while Islamic/Koranic education dominated the group of 502 respondents without formal education (52.8%).
Participants’ knowledge, awareness, practices related to Lassa fever infection prevention and control
Participants’ knowledge and practices related to Lassa fever infection control and prevention were evaluated, with knowledge categorised as low, moderate or high based on assessment question scores, and practices classified as poor, fair or good according to self-reported responses, as shown in Figure 2. Wealth categories were based on income and reported assets/material possessions.
Risk factors and determinants of Lassa fever transmission, prevention and control among participants
Chi-square and binary logistic regression analyses were conducted to examine the correlates of participants’ socio-demographic information, knowledge, awareness and behaviours, with their Lassa fever infection prevention and control practices.
In the adjusted model, participants from Kailahun district had higher odds for good/fair practices compared to those from Kenema (AOR = 1.57; 95% CI: 1.20–2.04; p = 0.001). Participants in the age groups of 30 years and above (30-39, 40-49, 50-59, and ≥ 60) were associated with good practices (AOR = 4.92; 95% CI: 1.67 - 14.49; p = 0.004; AOR = 7.75; 95% CI: 2.57 - 23.36; p = 0.001; AOR = 6.47; 95% 2.07 - 20.18; p = 0.001; AOR = 4.38; 95% 1.33 - 14.46; p = 0.015, respectively), compared to age groups below 30 years.
In the adjusted analysis, males were significantly less likely than females to practice infection control (AOR = 0.69; 95% CI: 0.51–0.94; p = 0.017) as shown in Table 1. After adjusting for all factors, teachers were more likely than farmers to practice infection control (AOR = 2.26; 95% CI: 1.05–4.87; p = 0.037). Marital status, household size and education were not significantly associated with infection prevention and control practices in the multivariate analysis. However, among those with formal education, participants with higher education were more likely to practice infection control compared to those with primary education (OR = 2.25; 95% CI: 1.23–4.13; p = 0.009).
Sources of information mattered, with health centres and workers being the most effective channels for promoting good practices (p = 0.001) as presented in Table S2 in the Online Supplementary Document. Knowledge of common Lassa fever symptoms was significantly associated with better infection control practices (p = 0.001). Participants who recognized multiple symptoms practiced better prevention, while those unfamiliar with the symptoms showed poorer adherence. Knowledge of Lassa fever transmission methods was a significant predictor of better infection control practices (p = 0.001), with participants who understood multiple transmission routes exhibiting better control practices.
There was a strong positive association between respondents’ knowledge and Lassa fever infection control practices (p = 0.001), indicating that higher knowledge levels were associated with better practices and lower knowledge levels with poorer practices. In the binary logistic regression analysis, participants with higher knowledge had over 11 times higher odds of practicing good or better infection control practices in both the unadjusted (OR = 11.41; 95% CI: 8.05–16.18; p = 0.001) and adjusted models (AOR = 10.64; 95% CI: 7.27–15.59; p = 0.001).
Participants in the higher wealth category reported good, hence better practices compared to those in the lower wealth category (p = 0.021). Participants in the moderate wealth category reportedly had significantly higher odds of practicing infection control than those in the low wealth category in both the unadjusted and unadjusted categorifies (OR = 1.51; 95% CI: 1.12–2.05; p = 0.008; and AOR = 1.60; 95% CI: 1.15–2.22; p = 0.05, respectively).
With regards to cultural or traditional practices, participants who reported following modern medical methods of handling the dead and burial recommendations had significantly higher odds of fair/good practice of Lassa fever control (Adjusted AOR = 8.69, 95% CI: 5.39–14.01; p = 0.001). Burial rituals showed significant association with infection control measures, with modern medical practices having better outcomes than traditional rituals involving close contact with the deceased (p = 0.001). The study also identified significant associations between gender roles related to caregiving, food handling, and rat hunting, with Lassa fever infection practices. Women were most often responsible for caregiving, with those in this role showing higher rates of good infection control practices than men (p = 0.013). Rat processing for consumption was frequently handled by boys, who scored lower adherence rates to infection control measures (p = 0.046). Rat hunting, primarily undertaken by boys and men, showed a significant association with infection control practices, with boys demonstrating poorer adherence (p = 0.001).
There were significant differences in key actions taken when participants suspected Lassa fever infection (p = 0.016); in response to a household member having symptoms (p = 0.001) and history of seeking medical care (p = 0.001), where those who had reported seeking medical care showed better practices than those who reported alternative treatment or caring options. The existence of geographic, financial constraints, healthcare provider and family support-related barriers were associated with poor practices as shown in Table S3 in the Online Supplementary Document.
DISCUSSION
Important findings made from this study on Lassa fever risk factors, transmission, prevention and control were: (1) Being male (particularly those engaged in rat hunting and rat meat processing); lower level of education; younger age; lower wealth status and engagement in traditional burials practices are the key risk factors for poor practices; (2) Awareness of recommended public health measures, knowledge of Lassa fever transmission routes, signs and symptoms are protective factors for good practices; (3) Reported barriers to good practices, or hindering access to healthcare, include geographic constraints (distance and transportation issues); financial limitations; perceived negative healthcare attitudes, and unsupportive family influences
The findings from this study are largely consistent with other existing study findings on risk factors and determinants of disease outbreak transmission, prevention and control. Collectively, the identified risk factors and determinants of diseases outcomes have been referred by WHO as the social or key determinants of health.14 This refers to a set of social and environmental factors existing at individual and societal or environmental level that continuously interact to shape observed health outcomes.15 These factors could include age, gender and gender roles, level of education, income or socio-economic position, geography, healthcare system, policies (including health, social, and micro and macro-economic) and political issues.16,17 The identified key research findings could be considered as interrelated and interacting to produce the observed outcome on practices. For instance, better education can improve one’s knowledge and perception of disease causation, transmission and treatment, thereby better informing their health behaviours and treatment choices. Better education can also be considered an important resource for creating chances for better income-generating opportunities and increasing one’s economic advantage. This can thereby improve one’s economic status or financial stability, leading to greater capacity and enhanced access to resources. Better income may imply better opportunities for education and healthcare access, and more power and control over living conditions and treatment options required to maintain or achieve better health outcomes.18,19 Consequently, according to this perspective, higher socio-economic status predicts better health outcomes, and where low socio-economic status is linked lesser control over the determinants of health, and consequently poor health outcomes.
In the context of social determinants of health, lower educational level can limit economic opportunities leading to lower wealth status and limited capacity to overcome barriers to good health. Lower levels of education can also contribute to inadequate understanding regarding Lassa fever transmission, potentially perpetuating traditional belief-based practices, such as unsafe burials, thereby increasing the risk of viral transmission. In low-income countries such as Sierra Leone, younger people often face disproportionate vulnerability due to economic instability, limited financial resources and reduced access to better living conditions and healthcare.17–19 All these circumstances can contribute to poor health practices. With regards to poor practices among males, existing research evidence suggest that males tend to exhibit poorer compliance with recommended health measures and often engage in riskier behaviours such as rat hunting and rat meat processing, compared to females.10,19
Given the consistency of the study findings with existing scientific and research evidence, and that Lassa fever remains a threatening endemic disease in the study area despite the continued interventions, more attention and investment are required for converting these findings into actionable recommendations in efforts to effectively interrupt the transmission.
Considering the research findings, the following recommendations are made:
-
Generation of contextual behavioural insights to complement quantitative research data findings. This will help to gain better community understanding of key perceptions, values and beliefs behind poor practices, apart from the already identified social determinants.
-
Utilization of context-aware research evidence to inform reprograming of behavioural change initiatives to better address the prevailing risk factors and determinants of Lassa fever transmission.
Study limitations
-
Measurement of some study variables, such as practices, relied on self-reported responses, thereby prone to potential residual confounding and biases, including recall bias. This could negatively impact the validity of the study findings.
-
The cross-sectional design of in this study may not capture temporal relationships between exposure and disease outcomes, which could be better expounded by a longitudinal study.
CONCLUSIONS
There is a complex interplay within and between risk factors and determinants of poor Lassa fever transmission, prevention and control practices. Therefore, effective control of Lassa fever transmission must leverage behavioural data insights to inform policy and programmatic decisions on Lassa fever response. Such interventions must adopt a comprehensive approach that considers the interconnectedness of the determinants of transmission and risk factors to sustain the prevention and control efforts.
Ethics statement
Ethical approval was obtained from the Government of Sierra Leone Office of Ethics and Scientific Review Committee approval 010/062024.
Data availability
The data and materials supporting the results or analyses of this paper will be made available from the corresponding author, Aminata Grace Kobie, Email: minatakobie@gmail.com on reasonable request to bona fide researchers.
Funding
No funding was received for the study.
Authorship contributions
Kobie AG: Conceptualization; Methodology; Investigation; Formal Analysis; Resources; Software; Visualization; Writing – original draft; Writing – review and editing. Okeibunor JC: Conceptualization; Methodology; Supervision; Validation; Writing – Review and Editing. Gonah L: Methodology; Data interpretation; Visualization; Validation; Writing – Review and Editing.
Disclosure of interest
The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
Additional material
The article contains additional information as an Online Supplementary Document.
Correspondence to:
-
Aminata Grace Kobie:
i. Texila American University, Guyana
ii. WHO Regional Office for Africa, Congo Brazzaville. Email: minatakobie@gmail.com -
Laston Gonah Department of Public Health, Faculty of Medicine and Health Sciences, Walter Sisulu University, Mthatha 5100, Eastern Cape, South Africa lgonah@wsu.ac.za; lggonah@gmail.com