Introduction
Sodium is an essential electrolyte in maintaining the body’s water balance. Hyponatraemia, defined as serum Na of lower than 135 mEq/L, is common across all clinical settings and needs to be managed effectively.1,2 Various pathophysiological mechanisms cause the imbalance between total body water and Na content, which are commonly classified according to volume status.3 For instance, syndrome of inappropriate antidiuretic hormone (SIADH) is a well-known diagnosis of exclusion for euvolemic hyponatraemia. Inappropriate secretion of anti-diuretic hormone (ADH) despite normal or increased plasma volume causes impaired water excretion by the kidney, leading to hyponatraemia.3
Neurological adaptations may result in chronic hyponatraemia, hence patients can be asymptomatic.4 Despite its relatively benign clinical presentation, mild hyponatremia is associated with fall in the elderly, leading to frequent admission and poor prognosis.5 In 2014, The European Renal Best Practice (ERBP) developed the Clinical Practice Guideline as a holistic compilation of the diagnostic approach and treatment of hyponatraemia.6 A review in 2017 also discussed common grounds and discrepancies between the United States and Europe guidelines.7 To date, there is no Clinical Practice Guideline (CPG) published in Malaysia for hyponatraemia.
In 2018, Tay CL et. al investigated the prevalence and causes of hyponatraemia among 253 geriatric patients attending primary care clinic.8 The result was less generalisable due to poor attendance of elderly patients. Another local study based in the emergency department only focused on the elderly population as well.9 There is a lack of studies on hospital-wide data for hyponatraemia. Therefore, we investigated the symptoms, causes and outcomes of hyponatraemia among hospitalised patients across different age groups with the aim to encourage further studies to provide an exhaustive overview of hyponatraemia in Malaysia, thus promoting the development of evidence-based local guidelines in the future.
Methods
This is a retrospective study conducted in Hospital Sultan Ismail (HSI), a tertiary government hospital in Johor with 700 beds and various subspecialties. Patients admitted with a Na level of <135mmol/L between January 2020 to December 2022 were identified through the electronic database. We aimed to study the clinical profile of hospital-wide patients with hyponatraemia. We investigated the symptoms and causes of hyponatraemia, and reviewed the management and outcomes of these patients.
Being the first hospital-wide local study on hyponatraemia, with uncertain prevalence among local patients across all age groups, we adopted a retrospective design to provide fundamental results for future prospective study. In view of the relatively small patient pool, we recruited all available cases instead of estimating a sample size to maximise our data. Patients with incomplete records or went home against medical advice were excluded to ensure data quality. We did not seek individual consent since existing anonymised patient records were utilised. The study was conducted with ethical principles outlined in the Declaration of Helsinki and Malaysian Good Clinical Practice Guideline. Ethical approval was granted by the Ministry of Health Malaysia (Ref:NMRR ID-23-00480-0GO (IIR) and University of Newcastle Medical School Ethics Committee, UK (Ref: 38882/2023).
The patients were admitted with hyponatraemia as the sole, or one of the diagnoses after assessing the symptoms, signs and investigation results. Data extracted included demography, comorbidities and regular medications. Relevant clinical symptoms were identified accordingly. Fluid status was determined by the formal documented physical examination.
The Na level on diagnosis was noted categorise the cases into mild (serum Na <135mmol/L), moderate (serum Na <130mmol/L) and severe (serum Na <120mmol/L) hyponatraemia.10 Variation exists regarding the definition of severe hyponatraemia. Na <120mmol/L was applied as literature mentioned symptoms do not usually appear until the plasma Na level drops below 120mmol/L.3,11 Data in the published literature suggests inpatient mortality rates of ≥50% for patients with Na level <120mmol/L as majority experienced significant acute progression of underlying illnesses.12–14
The following investigation results were documented: serum osmolality, urine osmolality, urine Na, serum glucose, lipid profile, serum protein, renal function test, liver function test, thyroid function test and serum morning cortisol. The causes and management of hyponatraemia were reviewed. The following outcomes were evaluated: treatment duration, change in Na level after 24 hours, complications of hyponatraemia and Na overcorrection. To ensure data consistency, all lab measurements were standardised across the study period.
All data were coded and imported anonymously into Microsoft Excel Worksheet. Data extraction method from patient record was discussed and agreed among all authors beforehand. Crosschecking was performed to ensure data accuracy. The collected data were kept on a password-protected database, which was deleted permanently after being copied to CDs. The CDs will be stored in a locked office of the investigators, and destroyed after a minimum of three years post study completion. SPSS version 29.0 was used for data analysis. Continuous data were expressed as mean and standard deviation. Categorical data were reported as frequencies and percentages. Chi-square test was applied for expected frequencies of >5, while Fisher’s exact test was applied for lower frequencies. The confidence interval was 95% and a p-value of <0.05 was considered significant.
Results
Patient characteristics
165 patients were identified during the study period (Table 1). The mean age was 64.4±12.9 years, with significantly larger proportion of elderly patients (p<0.001). There were more females than males (57.6% vs 42.2%, p=0.052). Majority have multiple comorbidities, most commonly hypertension (65.5%, n=108) and diabetes mellitus (47.3%, n=78). Almost one third were on medications associated with hyponatraemia. Diuretics was the most common medication group (18.2%, n=30), followed by PPI (3.6%, n=6) and anticonvulsants (3.0%, n=5).
As shown in Table 2, most patients had moderate (36.4%, n=60) or severe hyponatraemia (60.0%, n=99). The mean serum Na level on diagnosis was 117.5±7.7 mmol/L. Elderly patients formed the majority of severe cases (78.8%, p=0.002). There was no significant gender difference for each severity of hyponatraemia. 58.8% of patients were euvolemic, 37.6% were hypovolemic and 3.6% were hypervolemic (p<0.001). This distribution of patients according to volume status was consistent across all severities of hyponatraemia.
Symptoms of hyponatraemia
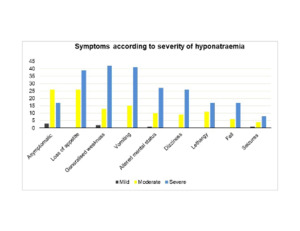
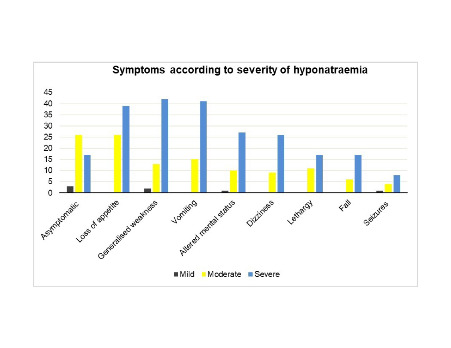
27.9% of patients did not present with symptoms of hyponatraemia. There were significantly less asymptomatic cases as the severity progressed (50% in mild, 43.3% in moderate and 17.2% in severe cases, p <0.001). Clinical presentations (Figure 1) included loss of appetite (39.4%), generalised weakness (34.5%), vomiting (33.9%), altered mental status (23.0%), dizziness (21.2%), lethargy (17.0%), fall (13.9%) and seizures (7.9%). Loss of appetite, generalised weakness and vomiting were the three most common manifestations in both moderate and severe hyponatraemia (Appendix 1, in the Online Supplementary Document). These were also the major clinical features in euvolemic and hypovolemic patients (Appendix 2, in the Online Supplementary Document). Among all symptoms, muscle weakness was significantly more prevalent in severe hyponatraemia (p=0.028).
Causes of hyponatraemia
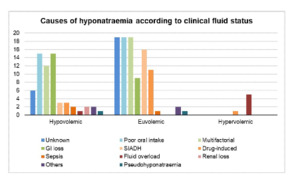
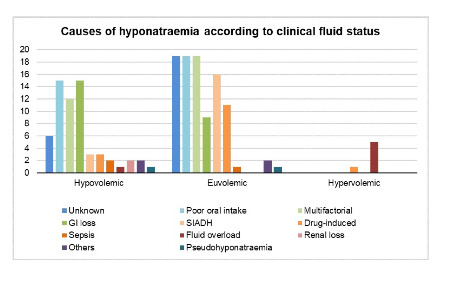
Figure 2 showed causes of hyponatraemia. 25 cases were discharged with unidentified cause. Poor oral intake (20.6%, n=34), multifactorial (18.8%, n=31) and gastrointestinal loss (14.5%, n=24) were the three most common causes. Among the 31 multifactorial cases, most patients were elderly (80.6% vs 19.4%, p=0.122) and have multiple comorbidities (77.4% vs 22.6%, p=0.624). Other causes of hyponatraemia included SIADH (11.5%), drug-induced (9.1%), sepsis (1.8%) and renal loss (1.2%). Hydrochlorothiazide was the cause in one third of drug-induced hyponatraemia. Poor oral intake accounted for most cases in both hypovolemic (24.2%, n=15) and euvolemic (19.6%, n=19) group. Among the euvolemic group (n= 97), SIADH was the third most common cause identified (16.5%, n=16) (Appendix 3, in the Online Supplementary Document).
Laboratory investigations for euvolemic patients (n=97) was reviewed. Only 17.5% patients had appropriate investigations for SIADH undertaken. All patients underwent renal function tests (urea and creatinine), 94.8% had serum osmolality (51.5% were calculated retrospectively by the researchers using Formula 1) and 73.2% had urine Na tested. However, only 38.1% of patients had urine osmolality results, 64.9% had thyroid function test, and 46.4% had early morning cortisol tested.
Formula 1
2 (serum Na + serum K) + serum glucose
Management of hyponatraemia
Table 3 and Appendix 4 (in the Online Supplementary Document) displayed the management of hyponatraemia. Majority had 0.9% sodium chloride (NaCl) infusion (89.7%, n=148). All 30 patients who received 3% hypertonic saline were having severe hyponatraemia. 83.3% of hypervolemic patients had fluid restriction, which also happened in 20.6% of euvolemic and 9.7% of hypovolemic patients. 29 patients have their regular medications withheld. Minority was given mist or oral NaCl as adjunctive treatment.
Treatment outcomes of hyponatraemia
The mean treatment duration was 8±6.4 days. It was 18 days for mild, 9 days for moderate and 7 days for severe hyponatraemia. Through Kruskal-Wallis test, we identified significant difference between patients with mild and severe hyponatraemia (18 vs 7 days, p<0.001) as well as between patients with mild and moderate hyponatraemia (18 vs 9 days, p=0.006). There was no significant difference on treatment duration among patients who received 3% hypertonic saline or otherwise (p=0.798). Most patients’ serum Na level increased after 24 hours, with 10.9% increased ≥10mmol/L (Appendix 5, in the Online Supplementary Document). Among these 18 patients, 5 (27.8%) received 3% hypertonic saline (p=0.487). 2 patients with severe hyponatraemia had coma, and 8 patients died. The mortality rate in the cohort was 4.85%. No osmotic demyelination syndrome was reported.
Discussion
Hyponatraemia is a complex condition, and the approach to its management should be tailored to the patient’s specific circumstances. A thorough evaluation is vital to address both the symptoms and the root cause of hyponatraemia in hospitalised adult patients.
Our study was the first to evaluate hyponatraemia among all hospitalised adult patients in a tertiary care centre. Loss of appetite, generalised weakness and vomiting were the main clinical presentations. The three major causes identified were poor oral intake, multifactorial and gastrointestinal loss. Most patients received 0.9% NaCl infusion. 10.9% of patients had Na level increment of ≥10mmol/L after 24 hours without complication.
Hyponatraemia was more common among our geriatric population. This is supported by a Doha study (mean age 56) and a Sub-Himalayan study (mean age 62).15,16 Ageing is accompanied with reduced total body water, higher sensitivity to osmotic stimuli and impaired regulation of water excretion.17 The elderly often have multiple comorbidities which correlates with polypharmacy, hence further contributing to hyponatraemia.17,18
Our patients consisted of more females than males. A Singapore study and another Indian study reported the same finding.19,20 In contrast, other studies reported male preponderance of cases.15,16,21–23 Despite this discrepancy, Xu Zhang et al.23 concluded that gender does not cause significant difference in the prevalence and severity of hyponatraemia. This was consistent with our study as there was no significant gender difference across each severity of hyponatraemia.
Our adult inpatients mostly had moderate or severe hyponatraemia, similar to the studies in Doha,15 Sub-Himalayan16 and India.20 Over half were euvolemic, which was consistent with most of the previous studies.19,21–23 There were almost one third of patients with asymptomatic hyponatraemia. This large proportion in view of the severity of cases suggests that hyponatraemia is a challenging condition to be identified and managed timely.
Among symptomatic patients, the frequency of all symptoms increased with increasing severity of hyponatraemia, as supported by Nilesh et al.21 Loss of appetite, generalised weakness and vomiting were the most common symptoms overall. This was consistent with a study where 27% complained of anorexia, and 17% presented with vomiting.16 Generalised weakness was among the leading symptoms of hyponatraemic patients admitted to the emergency department in Switzerland.24 Our patients also presented with altered mental status and seizures, which are common in hyponatraemia.19,24 According to Nikhil et al., 34% patients presented with altered sensorium.16 43% of patients in another study also had altered level of consciousness in the form of drowsiness, confusion, irrelevant talking or coma.21
In our study, among the 56 patients presented with vomiting, only 19 have GI loss-induced hyponatraemia. Similarly, among the 65 patients with loss of appetite, only 21 have poor oral intake identified as the cause. Loss of appetite as a symptom does not always link to poor oral intake as a cause, similar to vomiting. Clinicians should consider the possibility of hyponatraemia upon identifying these symptoms, and explore the variety of causes or co-existing pathologies behind.
Poor oral intake, multifactorial and GI loss were the leading causes of hypovolemic and euvolemic hyponatraemia. Similar study also reported poor oral intake as the main cause.25 In our study, most patients with poor oral intake were elderly. Their age-related physiological maladaptation predisposed them to depletional hyponatraemia. Patients with GI loss in our study mainly experienced vomiting or diarrhoea from acute gastroenteritis. As supported by Chatterjee et al., GI loss was also the leading hypovolemic hyponatraemia cause in their study.23 The disproportionate Na depletion compared to total water loss results in extracellular fluids contraction.26
A significant proportion of our cases were multifactorial, as supported by several other studies.27,28 Majority were elderly and those with multiple comorbidities. This could be contributed by age-related physiological maladaptation and polypharmacy as mentioned before.17,18 Hydrochlorothiazide caused the majority of drug-induced hyponatraemia by inhibiting NaCl reabsorption in the distal convoluted tubule.29 Careful use of thiazides is especially important in vulnerable populations such as those with increasing age, low body weight and hypokalaemia.29 Despite recent studies reporting SIADH as the leading cause of euvolemic hyponatraemia, it only ranked the third common in our study.27,30 This may be contributed by investigations shortfalls as reported by other papers.31
3% hypertonic saline is warranted in the presence of acute neurological signs to prevent hyponatraemic encephalopathy and cerebral oedema.32 Targeting correction rate of maximum 1 to 2 mmol/L/hour for the first 48 hours, Na level increment of 5 mmol/L is sufficient to provide significant clinical improvement.32,33 In our study, 9 patients with seizure and 23 patients with altered mental status did not receive rapid correction. Rapid correction should be offered unless concurrent underlying pathologies (epilepsy etc.) are suspected.32
Management should be tailored to the patient’s clinical volume status.6 Hypervolemic hyponatraemia mostly results from excessive water retention, hence can be addressed by fluid restriction and loop diuretics.4 0.9% NaCl infusion is the empirical therapy for hypovolemic hyponatraemia to replete the contracted ECF. In our study, 4 patients did not receive fluid therapy. 1 have chronic kidney disease, 2 have ascites whereas 1 did not have any contraindicating comorbidity. 6 patients had fluid restriction. Aside from a case of SIADH and another of fluid overload, the rest were incoherent with the standard treatment approach as fluid restriction has no proven benefit in hypovolaemic hyponatraemia.
Patients with SIADH should not receive 0.9% NaCl infusion as this isotonic fluid will promote net fluid shift into the intravascular space, thereby worsening hyponatraemia.34 In our study, 68% of SIADH cases received fluid infusion. This could be contributed by delayed diagnosis. This phenomenon again emphazied the need of a comprehensive local protocol to approach hyponatraemia.
The European Clinical Practice Guideline recommended Na correction of maximum 10 mmol/L during the first 24 hours.5 10.9% of our patients have ≥10mmol/L increment on the first day. Careful monitoring is crucial when handling future cases. Hyponatraemia is associated with prolonged hospital stay.35 In our study, patients with mild hyponatraemia had significantly longer duration of stay, which could be confounded by patient comorbidity and co-existing conditions. Studies reported that untreated hyponatraemia increases mortality risk due to neurological complications.36 5 of our patients died due to sepsis, 2 due to advanced cancer and 1 due to liver failure. More studies are warranted to further investigate the role of hyponatraemia in mortality.
Our findings were challenged by the relatively small sample size. The paucity of investigations reflected limitations in local protocol, which may lead to delayed diagnosis and mistreatment. Development of standardised protocol is much needed, especially for under-investigated causes like SIADH to ensure prompt diagnosis. Most cases did not have serum osmolality volume documented, which should ideally be done before starting treatment. Furthermore, our patients’ long-term outcomes could not be assessed as the study was retrospective.
Conclusions
Most patients were elderly with moderate or severe hyponatraemia. A large proportion of patients were asymptomatic. Loss of appetite, generalised weakness and vomiting were the main presentations. Besides poor oral intake and gastrointestinal loss, many cases of hyponatraemia were multifactorial. Most patients received 0.9% NaCl infusion, with 3% hypertonic saline offered in certain severe cases. Minority have ≥10mmol/L Na correction on the first day without complication.
This study should prompt the conduction of larger multicentre prospective studies to develop a standardised approach for hyponatraemia. Timely correction of sodium level can improve patient outcome especially among the elderly as they are the biggest group of patients that develop hyponatraemia. Patients on at-risk drugs should be monitored systematically to minimise incidence of hyponatraemia.
Funding
None.
Authorship contributions
All authors contributed to the manuscript and approved submission.
Disclosure of interest
The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
Additional material
Additional information is provided in the Online Supplementary Document.
Corresponding author:
Professor Edmund Ong Liang Chai
Address: 1, Jalan Sarjana 1, Educity, 79200 Iskandar Puteri, Johor, Malaysia
Email: e.l.c.ong@ncl.ac.uk
Acknowledgement
We would like to thank the Director General of the Ministry of Health, Malaysia for permission to undertake and publish this study.